Best Practices for Effective TLC Development, Chamber Saturation
Apr 30th 2025
Thin Layer Chromatography (TLC) is an essential technique for analyzing mixtures, checking purity, and monitoring reaction progress. When working with volatile organic solvents such as ethyl acetate, hexane, or dichloromethane, one often-overlooked but crucial step is chamber saturation before development. This step directly impacts the quality and reproducibility of your results.
Two major variables contribute to TLC separation variability:
- Ambient humidity: Water vapor in the lab environment can deactivate silanol groups on the normal unmodified phase silica gel surface. This reduces the stationary phase’s adsorption strength, leading to shifts in Rf values and less effective separation.
- Chamber saturation: Without proper vapor pressure equilibrium, volatile solvents evaporate unevenly during development. This causes spot distortion, inconsistent migration, and poor resolution.
In this blog, we review the effect of chamber saturation on thin-layer chromatography (TLC) development—an often underestimated factor that significantly impacts resolution and reproducibility.
Step 1: Pre-Saturate the Chamber Using a Blank TLC Plate
Begin by adding a shallow layer of mobile phase (typically 0.5–1 cm) into the bottom of the chamber. Next, insert a blank TLC plate (a plate without any sample spots) vertically into the chamber. This plate increases the surface area for solvent evaporation, which helps to quickly build up solvent vapor inside the sealed chamber.
Seal the chamber tightly with a lid to trap the vapors and begin establishing vapor pressure equilibrium.
Let the chamber equilibrate until the mobile phase rises close to the top of the blank TLC plate. This ensures maximum vapor saturation and confirms consistent solvent behavior inside the chamber.
Once the solvent front reaches near the top edge of the blank plate, remove the strip and immediately proceed to insert the sample-spotted TLC plate (see Step 2 below), minimizing exposure to open air.

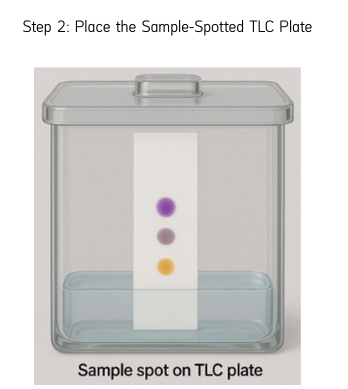
Step 2: Place the Sample-Spotted TLC Plate
Once saturation is achieved, remove the blank TLC plate and quickly place the sample-spotted TLC plate without disturbing the vapor-rich environment. Ensure that the spots are above the solvent level.
Seal the chamber again and allow the mobile phase to ascend. When development is complete, remove the plate, mark the solvent front, and allow it to dry before visualization or analysis.
Why Saturate the Chamber?
Volatile solvents readily evaporate, and without a saturated environment, the mobile phase can evaporate during the development process. This leads to poor or uneven development, distorted Rf values, and tailing or streaked spots.
Saturating the chamber builds vapor pressure equilibrium, minimizing solvent loss from the developing TLC plate and ensuring uniform upward flow of the mobile phase.
Vapor pressure equilibrium: A balanced state where the rate of solvent evaporation equals the rate of condensation within the closed chamber.
This equilibrium is key to a uniform capillary action on the TLC plate. It slows down solvent evaporation from the developing plate’s surface, allowing even migration of compounds and sharper, better-separated spots.
Here’s what happens during saturation:
-
Evaporation: The mobile phase (solvent) at the bottom of the chamber starts to evaporate, especially if it’s volatile.
-
Vapor Build-Up: As more solvent evaporates, the chamber fills with solvent vapor.
-
Condensation: Some of that vapor touches the cooler surfaces inside the chamber and condenses back into liquid droplets.
This cycle of evaporation and condensation continues until vapor pressure equilibrium is reached—meaning the rate of evaporation equals the rate of condensation.
Why is this helpful?
-
It stabilizes the atmosphere inside the chamber.
-
Prevents further net loss of solvent from the developing TLC plate.
-
Ensures even and reproducible migration of sample components up the plate.
Summary
-
Use a blank TLC plate to pre-saturate the chamber.
-
Keep the chamber sealed with a lid during and after solvent addition.
-
This method ensures accurate Rf values, clean separations, and reproducibility, especially when using volatile organic solvents.
For any TLC analysis involving volatiles—don’t skip the saturation step. It’s a small adjustment that leads to significantly better chromatography.

