TLC Material Selection
Jun 4th 2025
In radiopharmaceutical quality control (QC), thin-layer chromatography (TLC) is a critical technique. It provides a simple, rapid, and cost-effective means to assess the radiochemical purity of radiopharmaceuticals. This ensures patient safety and accurate diagnostic or therapeutic outcomes by verifying that the radioactive isotope is correctly bound to the intended pharmaceutical compound.
TLC helps identify and quantify:
- Free Radioisotopes: Unbound radioactive material.
- Hydrolyzed / Colloidal Forms: Undesirable chemical forms of the radioisotope.
- Labeled impurities: Radioactive contaminants in the radiopharmaceutical.
Achieving reliable QC results relies on choosing the right TLC material for the specific radiopharmaceutical and separation requirements. This post compares three commonly used TLC materials: Whatman cellulose paper, Baker-Flex aluminum oxide and Molpure ITLC SG.
TLC Materials Overview
Let’s introduce the three main TLC materials used in radiopharmaceutical QC:
- Whatman Cellulose Paper: A paper-based TLC media primarily used for partition chromatography.
- Molpure ITLC SG paper: A silica gel impregnated glass microfiber sheet used for adsorption chromatography, designed for rapid and reliable separations in radiopharmaceutical QC.
- Baker-Flex Aluminum Oxide: An aluminum oxide-based TLC plate. Suitable for adsorption chromatography.
Chromatographic Mechanism: Partition vs. Adsorption
Understanding the underlying chromatographic mechanisms is crucial for selecting the appropriate TLC material.
Partition Chromatography: Separation is based on the differential partitioning of analytes between a stationary liquid phase (e.g. water absorbed on cellulose) and a moving liquid phase (mobile phase). Whatman cellulose paper (Whatman 1, 3MM, 31ET, etc.) primarily utilizes this mechanism.
Adsorption Chromatography: Separation is based on the differential adsorption of analytes onto a solid stationary phase (e.g. silica gel or aluminum oxide). Molpure ITLC SG (and SA ) and Baker -Flex aluminum primarily utilize this mechanism.
The Three Main Radiopharmacy QC TLC Materials
|
Feature |
Whatman Chr |
Baker-Flex Aluminum Oxide |
Molpure ITLC |
|
Stationary Phase |
Cellulose (pure cotton linters/paper) |
Aluminum Oxide (Al₂O₃) |
Silica Gel (SiO₂) typically impregnated onto glass microfiber |
|
Primary Mechanism |
Partition Chromatography |
Adsorption Chromatography |
Adsorption Chromatography |
|
Pore Size/Flow Rate |
General applications, medium/heavy solute loadings. |
Generally uniform coating thickness for considerable sample capacity. |
Varies by specific Molpure product (e.g., SG TLC-100 is "light" loading, SG TLC-400 is "medium" loading), impacting middle Rfs . |
|
Typical Radiopharm. QC Uses |
Used for hydrophilic/water-soluble compounds |
Traditionally the gold standard for lipophilic/protein-bound ⁹⁹ᵐTc-agents for colloid assessment. |
The most versatile and widely used for general radiopharmaceutical QC, especially for free pertechnetate and colloid assessment. |
|
Key Separation |
Separates based on hydrophilicity/aqueous solubility. Unbound pertechnetate often has a high Rf in aqueous/saline systems, while many protein/peptide complexes stay at the origin. |
Separates based on polarity/adsorption strength. Highly polar, insoluble colloids stay at the origin, while less polar radiopharmaceuticals and free pertechnetate migrate. |
Highly versatile for both polar and non-polar separations depending on mobile phase. Excellent for differentiating free pertechnetate and colloids from the desired radiopharmaceutical. |
|
Physical Form |
Paper sheets or strips. Generally more flexible but can be more fragile and easily damaged (e.g., tearing, wrinkling). |
Flexible plastic or aluminum sheets with an aluminum oxide coating. Can sometimes be prone to cracking/crumbling of the coating when cut. |
Glass microfiber sheets impregnated with silica gel. Often pre-cut and marked, generally robust for handling and cutting. |
|
Preparation/Activation |
Generally used as is, but can be pre-washed or modified for specific applications. |
Some specific types or older protocols might recommend heat activation (e.g., oven baking) and storage in a desiccator, though this varies. |
Generally ready-to-use with minimal pre-treatment required. Proper storage is always recommended. |
|
Reproducibility |
Good |
Generally good. |
Excellent, often with tight specifications for batch-to-batch consistency. |
|
Typical Mobile Phase |
Aqueous solutions (e.g., saline, water), or polar organic solvents |
Highly polar organic solvents like 95-100% ethanol. |
Wide range of solvents: acetone, MEK, saline, ethanol, mixtures thereof. |
|
Advantages |
Cost-effective, good for highly polar separations, widely available in general lab settings. |
Historically validated by manufacturers for specific ⁹⁹ᵐTc-agents (like Sestamibi), direct assessment of colloidal impurity. |
Highly versatile, widely used, often pre-cut/pre-marked for convenience, excellent for a broad range of radiopharmaceuticals. Can be tailored for various separations via different solvent systems. |
|
Disadvantages |
Slower development times compared to SG ITLC, less physically robust, often requires two systems for complete QC, can show tailing for some compounds. May not effectively separate lipophilic compounds. |
Limited to specific separations (colloid assessment for lipophilic agents), can be fragile when cutting, might require specific handling. |
Less ideal for highly polar or very hydrophilic compounds where cellulose might provide better resolution (though often a different solvent system can compensate). |
TLC in Action: 99mTc-Sestamibi Example
Step-by-Step Procedure
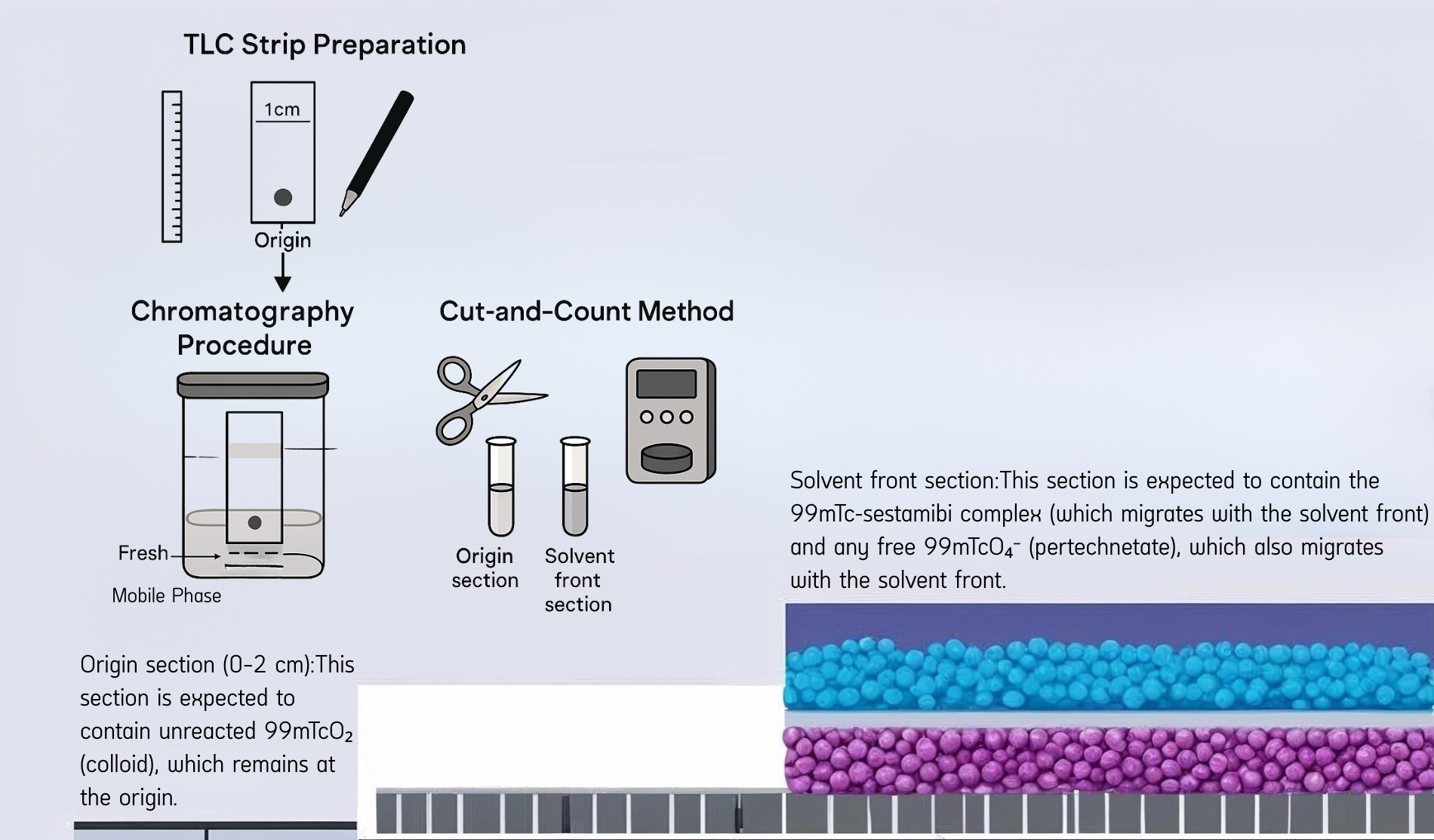
- TLC Strip Preparation
- Mark the origin at 1 cm from the bottom of the strip.
- Spot the radiopharmaceutical sample (e.g., 99mTc-sestamibi) at the origin.
- Chromatography Development
- Place the strip vertically into a sealed chromatography chamber containing the mobile phase.
- The solvent moves upward by capillary action, carrying components based on their interaction with the stationary phase.
- Cut-and-Count Method
- Once the solvent front has migrated sufficiently, cut the strip into two parts:
- Origin section (0–2 cm): Expected to retain colloidal 99mTcO₂, which does not migrate.
- Solvent front section: Contains 99mTc-sestamibi and free pertechnetate (99mTcO₄⁻), both of which travel with the solvent front.
- Measure radioactivity in each section using a gamma counter.
To evaluate TLC material performance, multiple trials were conducted using two Molpure ITLC SG formats and a Whatman 31ET (Biodex Pink) strip. The mobile phase used was Acetone.
|
Trial |
Material |
Top (cpm) |
Bottom (cpm) |
% Tagged |
Run Time |
|
Trial 1 |
Molpure TL-100 |
30,519 |
733 |
97.65% |
1 min 40 sec |
|
Molpure TL-400 |
33,909 |
1,188 |
96.62% |
2 min 03 sec |
|
|
Trial 2 |
Molpure TL-100 |
37,902 |
1,320 |
96.63% |
1 min 35 sec |
|
Molpure TL-400 |
37,632 |
946 |
97.55% |
2 min 12 sec |
|
|
Trial 3 |
Molpure TL-100 |
19,212 |
844 |
95.79% |
1 min 32 sec |
|
Molpure TL-400 |
19,141 |
957 |
95.24% |
1 min 49 sec |
|
|
Whatman 31ET |
25,736 |
1,933 |
93.01% |
1 min 03 sec |
Practical Guidance: Selecting the Right TLC Material
The choice of TLC materials depends on several factors:
- Radiopharmaceutical: Consider the chemical properties of the radiopharmaceutical being analyzed.
- Separation Requirements: Identify the specific impurities or components that need to be separated.
- Mobile Phase Compatibility: Ensure the TLC material is compatible with intended mobile phase.
- Regulatory Requirements: Adhere to relevant regulatory guidelines and compendial methods.
General Recommendations:
- Whatman Cellulose Paper: Best suited for separating polar compounds like free pertechnetate or iodide using polar mobile phases (e.g. Saline).
- Baker-Flex Aluminum Oxide: Ideal for separating non-polar compounds or lipophilic impurities using less polar mobile phases (e.g. ethyl acetate).
- Molpure ITLC Silica Gel (SG and SA): Specially designed for radiopharmaceutical QC, offering rapid and reliable separations for common radiochemical impurities. It provides a good balance for many applications where speed and optimization are critical.
Ultimately, validation studies should be performed to ensure the chosen TLC material and method are suitable for the intended purpose.

